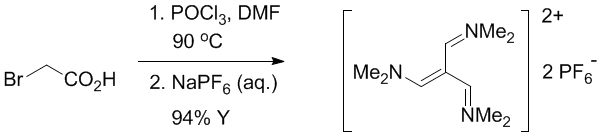
Anhydrous DMF 160mL in a 0.5L round flask was cooled on ice bath and neat POCl3 40 mL (436mmol) was added greadually over 10 min period (exothermic). The mixture was warmed up to RT, bromoacetic acid solid 19.00g (136.7 mmol, Aldrich 99%+) was added quickly in one portion (hygroscopic, wear gloves – bromoacetic acid and POCl3 are nasty). The flask was equipped with a reflux condenser and the mixture was stirred under Ar on oil bath at 90C for 9 hours. The mixture turned orange, with a slow CO2 evolution. The mixture was cooled to RT, DMF was distilled out on highvac (RT to 90C at 0.5 Torr) and the obtained thick residue was treated with ice-cold water 100mL on a sonicator bath. An exothermic hydrolysis commenced. When all residue dissolved and the mixture cooled to RT, solid sodium hexafluorophosphate 46.2g (275 mmol) was added in one portion followed by some additional water (50 mL). The mixture was shaken vigorously for 10 min, the precipitated product was collected using a large Buchner funnel. The solid cake was compressed on the frit, washed with ice-cold water (5x20mL), dried by suction and then on highvac.
Y=61.05g (94%) of a yellowish-white solid
1H(d6-DMSO, 400MHz): 8.439(s, 3H), 3.535(s, 9H), 3.376(s, 9H)
Note 1: The formylation is complete in 6 hours at 90C. KPF6 is probably a better source of hexafluorophosphate because unlike the sodium salt it does not hydrolyse readily, does not etch glass and is non-hygroscopic. Aqueous HPF6 can be also used. It is important to remove most DMF from the reaction mixture, this takes some time (I used a rotovap hooked to oil pump).
Note 2: The product can be re-crystallised to analytical purity from MeCN + some antisolvent (THF) but the crude material is suitable for most purposes.
Note 3: Bromoacetic acid can be replaced with bromoacetyl chloride or bromide – in such case the POCl3 amount can be reduced by one equivalent